Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 03-SECTION II
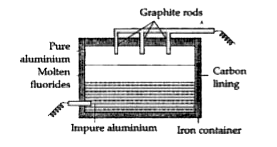
- The following question relate to the extraction of aluminium by electr...
Text Solution
|
- The following question relate to the extraction of aluminium by electr...
Text Solution
|
- The following question relate to the extraction of aluminium by electr...
Text Solution
|
- The following question is related to Iron: Name the acid with which ...
Text Solution
|
- The following question are related to Iron: Name an alloy of iron an...
Text Solution
|
- The following question are related to Iron: Name the process by whic...
Text Solution
|
- Complete the following statements using proper words: In a thermit...
Text Solution
|
- Complete the following statements using proper words: In dry cells,...
Text Solution
|
- Use the letters only written in the Periodic Table given below to ans...
Text Solution
|
- Use the letters only written in the Periodic Table given below to ans...
Text Solution
|
- Use the letters only written in the Periodic Table given below to ans...
Text Solution
|
- Use the letters only written in the Periodic Table given below to ans...
Text Solution
|
- Arrange the following as per the instructions given in the brackets: ...
Text Solution
|
- Arrange the following as per the instructions given in the brackets: ...
Text Solution
|
- Arrange the following as per the instructions given in the brackets :...
Text Solution
|
- Arrange the following as per instructions given in the brackets: Cl,...
Text Solution
|
- By drawing an electron dot diagram, show the lone pair effect leading...
Text Solution
|
- Identify the acid which matches the following description : Give bal...
Text Solution
|
- Name a yellow monoxide that dissolves in hot and concentrated alkali. ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|