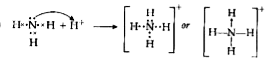Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 03-SECTION II
- Arrange the following as per the instructions given in the brackets :...
Text Solution
|
- Arrange the following as per instructions given in the brackets: Cl,...
Text Solution
|
- By drawing an electron dot diagram, show the lone pair effect leading...
Text Solution
|
- Identify the acid which matches the following description : Give bal...
Text Solution
|
- Name a yellow monoxide that dissolves in hot and concentrated alkali. ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|
- Why is (a) concentrated sulphuric acid kept in air tight bottles ?
Text Solution
|
- Why is H2SO4 not a drying agent for H2S ?
Text Solution
|
- Copy and Complete the following table which refers to two practical ap...
Text Solution
|
- The questions below are related to the manufacture of ammonia. Name ...
Text Solution
|
- The questions below are related to the manufacture of ammonia. In wh...
Text Solution
|
- The questions below are related to the manufacture of ammonia. Name ...
Text Solution
|
- The questions below are related to the manufacture of ammonia. Give ...
Text Solution
|
- Consider the following reaction and based on the reaction answer the q...
Text Solution
|
- Consider the following reaction and based on the reaction answer the q...
Text Solution
|
- Consider the following reaction and based on the reaction answer the q...
Text Solution
|
- Consider the following reaction and based on the reaction answer the q...
Text Solution
|
- Correct the following if required: HNO3 is a strong reducing agent.
Text Solution
|