Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 5-Section II
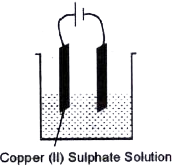
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- The following questions are pertaining to the laboratory preparation o...
Text Solution
|
- The following questions are pertaining to the laboratory preparation o...
Text Solution
|
- The following questions are pertaining to the laboratory preparation o...
Text Solution
|
- The following questions are pertaining to the laboratory preparation o...
Text Solution
|
- The elements of one short period of the periodic table are given below...
Text Solution
|
- The elements of one short period of the periodic table are given below...
Text Solution
|
- The elements of one short period of the periodic table are given below...
Text Solution
|
- The elements of one short period of the periodic table are given below...
Text Solution
|
- The elements of one short period of the periodic table are given below...
Text Solution
|
- Match the salts given in Column I with their . method of preparation g...
Text Solution
|
- A gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour...
Text Solution
|
- Find the total percentage of magnesium in magnesium nitrate crystals [...
Text Solution
|
- The following table shows the tests a student performed on four differ...
Text Solution
|
- Name a metal which is found abundantly in the earth's crust.
Text Solution
|
- State one observation: A zinc granule is added to copper sulphate solu...
Text Solution
|
- Give reasons why copper is used to make hot water tanks and not steel ...
Text Solution
|
- Write the name and formula of an ore of aluminium.
Text Solution
|