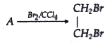Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2016-SECTION-II
- Give the structural formula of the following: ethanoic acid
Text Solution
|
- Give the structural formulae of the following: Butan-2-ol
Text Solution
|
- Equation for the reaction when compound A is bubbled through bromine d...
Text Solution
|
- Equation for the reaction when compound A is bubbled through bromine d...
Text Solution
|
- Fill in the blanks using the appropriate words given below: (Sulphur...
Text Solution
|
- Fill in the blanks using the appropriate words given below: (Sulphur...
Text Solution
|
- State your observations when : Dilute hydrochloric acid reacts with ...
Text Solution
|
- State your observations when : Dilute hydrochloric acid reacts with ...
Text Solution
|
- State your observations when ammonium hydroxide solution is added drop...
Text Solution
|
- State your observations when ammonium hydroxide solution is added drop...
Text Solution
|
- Write equations for the reactions taking place at the two electrodes (...
Text Solution
|
- Write equations for the reactions taking place at the two electrodes (...
Text Solution
|
- Name the product formed at the anode during the electrolysis of acidif...
Text Solution
|
- Name the metallic ions that should be present in the electrolyte when ...
Text Solution
|
- A gas cylinder contains 12 xx 10^(24) molecules of oxygen gas. If Av...
Text Solution
|
- A gas cylinder contains 12 xx 10^(24) molecules of oxygen gas. If Av...
Text Solution
|
- A gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour...
Text Solution
|
- The equation 4NH3 + 5O2 rarr 4NO + 6H2O, represents the catalytic oxid...
Text Solution
|
- By drawing an electron dot diagram show the formation of Ammonium Ion ...
Text Solution
|
- Name the gas evolved when the following mixture is heated : Calcium ...
Text Solution
|