Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2012-PART-II (SECTION-A)
- A solution of urea in water has a boiling point of 100*18^@C. Calculat...
Text Solution
|
- A solution of lactose containing 8.45 g of lactose in 100g of water h...
Text Solution
|
- The molecular weight of H2S is more than that of H2O, but H2S is a gas...
Text Solution
|
- When potassium cyanide reacts with water, will the resulting solution ...
Text Solution
|
- What is the hybridization of the carbon atom in ethyne molecule ? What...
Text Solution
|
- State and explain the second law of thermodynamics.
Text Solution
|
- Calculate the maximum work that can be obtained from the given electr...
Text Solution
|
- To precipitate group III cations NH4Cl should be added to the solution...
Text Solution
|
- A study of chemical kinetics of the reaction A + B to products, gave t...
Text Solution
|
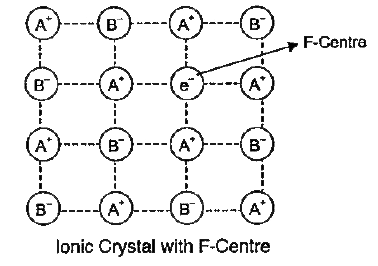
- What are F-centres in an ionic crystal ?
Text Solution
|
- Why solids with F-centres are paramagnetic?
Text Solution
|
- The central atom of methane and water is in the same state of hybridiz...
Text Solution
|
- The conductivity of 0*2M " KCl solution is " 3 Xx 10^(-2) ohm^(-1) cm^...
Text Solution
|
- Draw the valence shell molecular orbital diagram of oxygen molecule an...
Text Solution
|
- Calculate the solubility of lead chloride in water, if its solubility ...
Text Solution
|
- For a crystal of diamond, state : The hybridization of the carbon at...
Text Solution
|
- For a crystal of diamond, state : The coordination number of each ca...
Text Solution
|
- For a crystal of diamond, state : The type of lattice in which it cr...
Text Solution
|
- For a crystal of diamond, state : The number of carbon atoms present...
Text Solution
|