Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER 2013
ICSE|Exercise PART-II (SECTION-B)|16 VideosSAMPLE PAPER 2013
ICSE|Exercise PART-II (SECTION-C)|17 VideosSAMPLE PAPER 2013
ICSE|Exercise PART-II (SECTION-C)|17 VideosSAMPLE PAPER 2012
ICSE|Exercise PART-II (SECTION-C)|21 VideosSAMPLE PAPER 2014
ICSE|Exercise PART-II (SECTION -C)|21 Videos
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2013-PART-II (SECTION-A)
- Ethylene glycol is used as an antifreeze agent. Calculate the amount o...
Text Solution
|
- The freezing point of a solution containing 0*3 gms of acetic acid in ...
Text Solution
|
- Name the law or principle confirmed by the following observations : ...
Text Solution
|
- Name the law or principle confirmed by the following observations : ...
Text Solution
|
- Arrange Ag, Cr and Hg metals in the increasing order of reducing power...
Text Solution
|
- In a first order reaction, 10% of the reactant is consumed in 25 minut...
Text Solution
|
- In a first order reaction, 10% of the reactant is consumed in 25 minut...
Text Solution
|
- Explain giving reasons why (Give equations in support of your answer) ...
Text Solution
|
- Explain giving reasons why (Give equations in support of your answer) ...
Text Solution
|
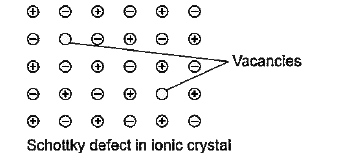
- What is Schottky defect in a solid ?
Text Solution
|
- A bcc element (atomic mass 65) has cell edge of 420 pm. Calculate its ...
Text Solution
|
- The rate of the reaction H2 + I2 to 2HI " is given by" :^(**"**) Rat...
Text Solution
|
- According to Lewis concept of acids and bases, ethers are
Text Solution
|
- The solubility of Ag2CrO4 " at " 25^@C " is " 8*0 xx10^(-5) moles/litr...
Text Solution
|
- Define molar conductance of a solution. State its unit. How is it rela...
Text Solution
|
- Calculate the value of E("cell") at 298 K for the following cell: ...
Text Solution
|
- Calculate the degree of hydrolysis of 0*2 (M) sodium acetate solution....
Text Solution
|
- Explain why high pressure is used in the manufacture of ammonia by Hab...
Text Solution
|