Similar Questions
Explore conceptually related problems
Recommended Questions
- In an atom the number of electrons in M - shell is equal to the number...
Text Solution
|
- The possible number of orientations of a sun-shell is (2 l + 1) The po...
Text Solution
|
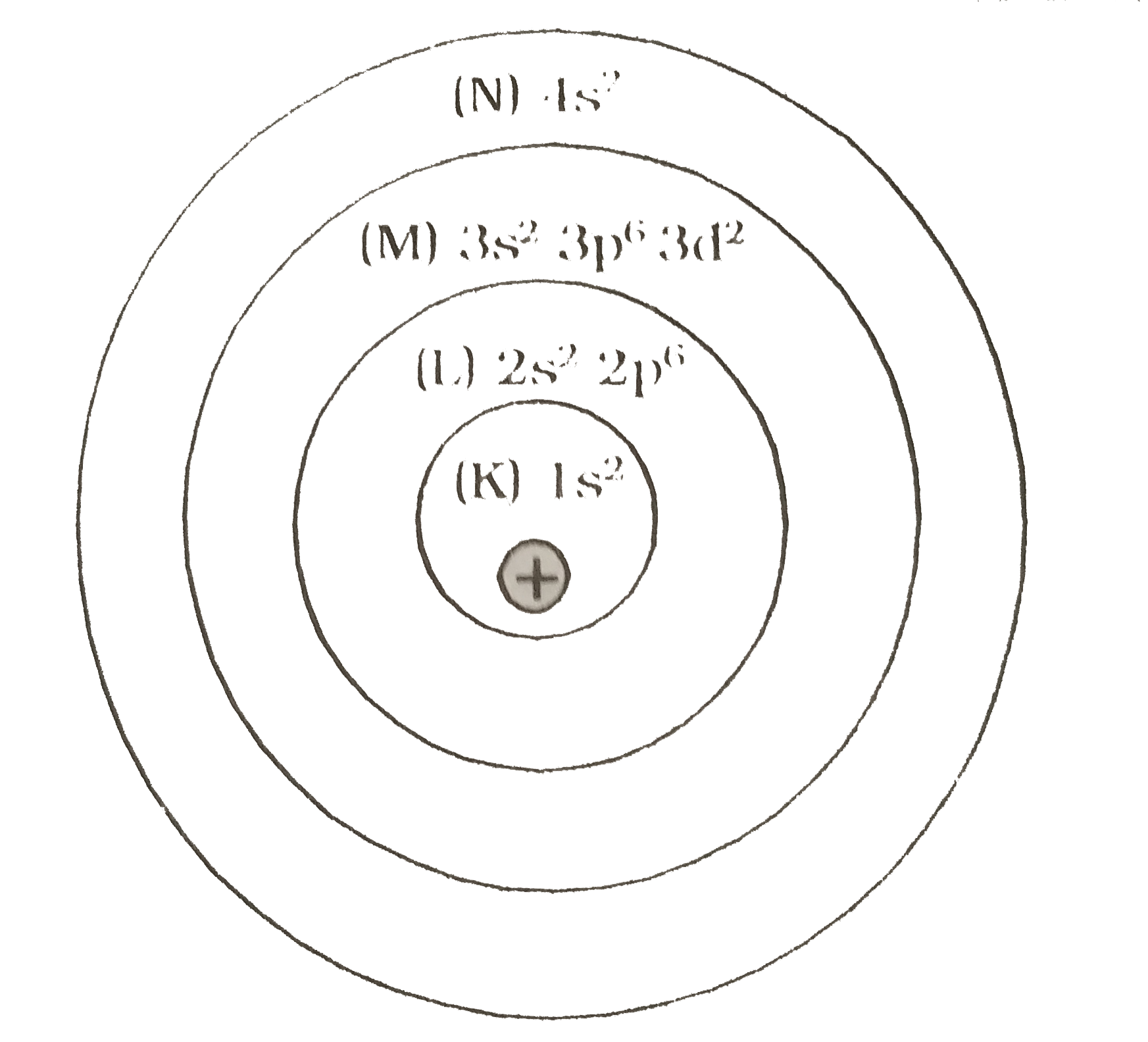
- An element has 2 electrons in K shell, 8 electrons in L shell, 13 elec...
Text Solution
|
- An atom has 2 electrons in K shell, 8 electrons in L shell and 6 elect...
Text Solution
|
- In an atom the number of electrons in M - shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in M - shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in M - shell is equal to the number...
Text Solution
|
- L कोश में इलेक्ट्रॉनों की संख्या होती है -
Text Solution
|
- An element has 2 electrons in its K-shell. 8 electrons in L-shell, 13 ...
Text Solution
|