Similar Questions
Explore conceptually related problems
Recommended Questions
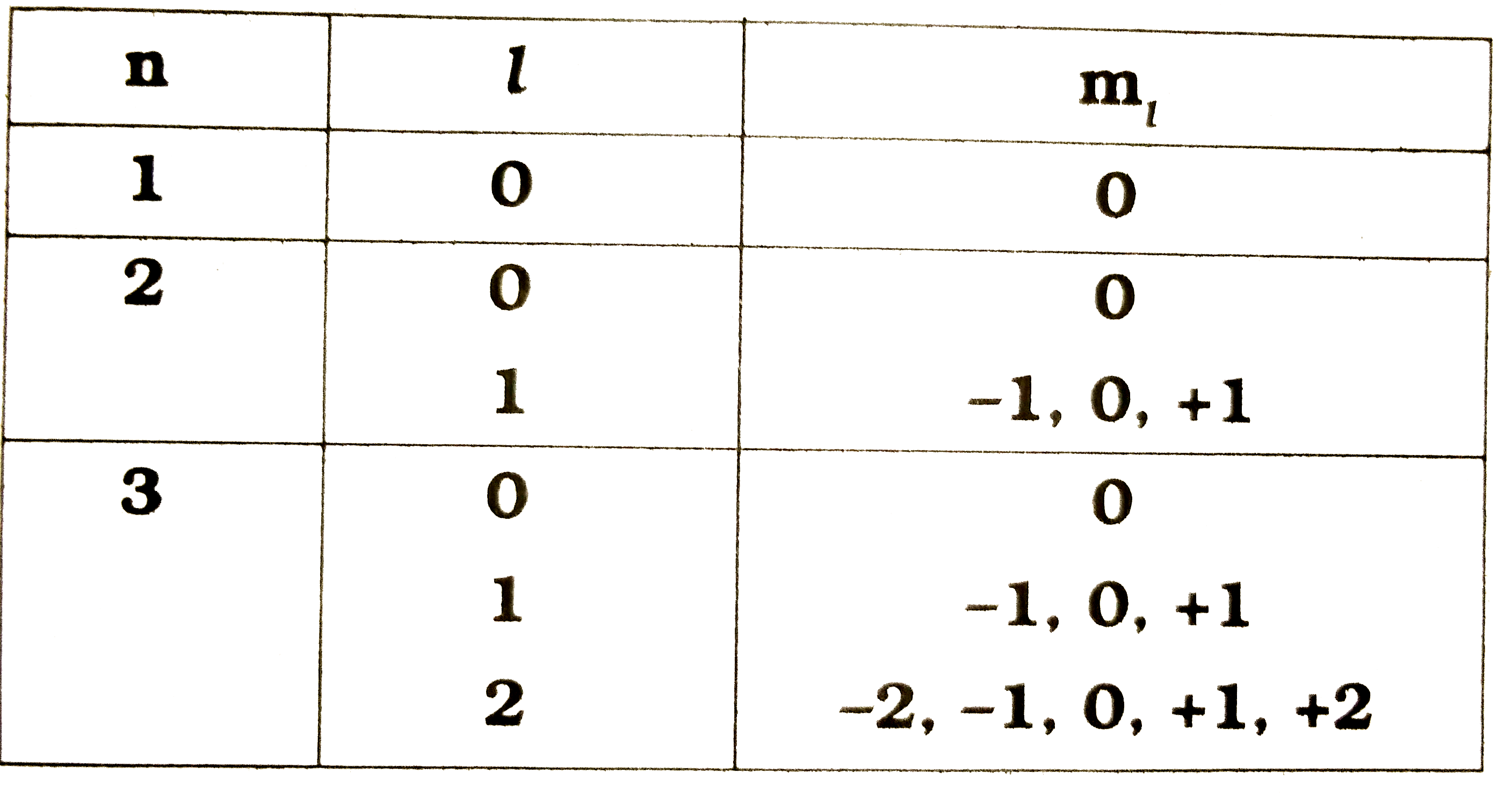
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|
- The set of quantum numbers, n = 3, l = 2, m(l) = 0
Text Solution
|
- The set of quantum numbers, n = 2, l = 2, m(l) = 0 :
Text Solution
|
- If a quantum number l has value of 2, what are the permitted values of...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|