Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-BASICS IN CHEMISTRY-QUESTION BANK
- Calculate the mass of 2.5 gram atom of magnesium.
Text Solution
|
- How many gram molecules are present in 4.9g of H2SO4?
Text Solution
|
- Why do we regard the gaseous state of water as vapours while that ammo...
Text Solution
|
- An element has fractional atomic mass. What does this indicate?
Text Solution
|
- When 4.2 g NaHCO3 is added to a solution of CH3COOH, weighing 10 g, it...
Text Solution
|
- Copper sulphate crystals contain 25.45% Cu and 36.07% H2O. If law of c...
Text Solution
|
- 10 ml of H2 contain 2000 molecules under certain temperature and press...
Text Solution
|
- One volume of hydrogen combines with sulphur to produce one volume of ...
Text Solution
|
- A flask P contains 0.5 mole of oxygen gas. Another flask Q contains 0....
Text Solution
|
- From 200mg of CO2, 10^21 molecules are removed. How many moles of CO2 ...
Text Solution
|
- How many molecules of CO2 are present in one litre of air containing 0...
Text Solution
|
- On analyzing an impure sample of sodium chloride, the percentage of ch...
Text Solution
|
- Copper gives two oxides. On heating one gram of each in hydrogen, we g...
Text Solution
|
- The average atomic mass of copper is 63.5u. It exists as two isotopes ...
Text Solution
|
- P and Q are two elements which form P2Q3 and PQ2 molecules. If 0.15g m...
Text Solution
|
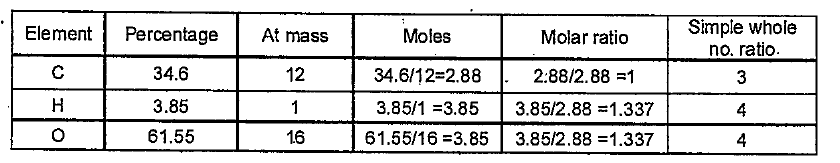
- A compound on analysis was found to contain C=34.6%, H=3.85% and O = 6...
Text Solution
|
- On heating 1.763 g of hydrated BaCI2 to dryness, 1.505g of anhydrous s...
Text Solution
|
- 1.0g of Mg is burnt in a closed vessel which contains 0.5g of O2. Whic...
Text Solution
|
- A mixture of FeO and Fe3O4 when heated in air to a constant weight gai...
Text Solution
|
- Butyric acid contains C,H,O elements. A4.24 mg sample of butyric acid ...
Text Solution
|