Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
PATHFINDER-ATOMIC STRUCTURE-QUESTION BANK
- The ionisation Energy of He^+ ion is 19.6x10^-18 J atom^1. Find the e...
Text Solution
|
- The atomic number of elements A and B is 9 and 17 respectively. Why is...
Text Solution
|
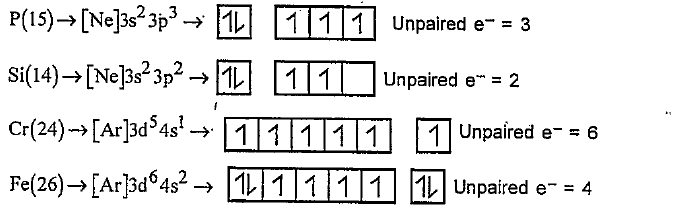
- Indicate the number of unpaired electrons in a) P(15), b) Si(14), c) C...
Text Solution
|
- Give the difference between orbit and orbital.
Text Solution
|
- Write the isoelectronic species of Na^+, K^+, Mg^+2, Ca^+2, S^-2, Ar.
Text Solution
|
- What should be the ratio of velocities of CH4 molecule and O2 molecule...
Text Solution
|
- The mass of an electron is 9.1xx10^-31kg. If its K.E. is 5xx10^-25J. C...
Text Solution
|
- An electron in a Bohr orbit of hydrogen atom in quantum level n2 has a...
Text Solution
|
- Match the column:
Text Solution
|
- Match the column:
Text Solution
|
- A subshell with n = 6 and / = 3 is designated as .
Text Solution
|
- Find the change in velocity of an electron which has been excited from...
Text Solution
|
- A bulb emits light of wavelength 4500A^@.The bulb is rated as 150 watt...
Text Solution
|
- A photon of 300 nm is absorbed by a gas and then re emitted as two pho...
Text Solution
|
- An electron is moving in 3rd orbit of Li^(+2) calculate Radius
Text Solution
|
- An electron is moving in 3rd orbit of Li^(+2) calculate velocity
Text Solution
|
- An electron is moving in 3rd orbit of Li^(+2) calculate potential ene...
Text Solution
|
- An electron is moving in 3rd orbit of Li^(+2) calculate kinetic energ...
Text Solution
|
- An electron is moving in 3rd orbit of Li^(+2) calculate energy
Text Solution
|
- The energy of second orbit of hydrogen is equal to the energy of
Text Solution
|