Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-PERIODIC CLASSIFICATION OF ELEMENTS AND PERIODIC PROPERTIES-QUESTION BANK
- What are 'transition' elements?
Text Solution
|
- What do you mean by 'inner-transition elements'?
Text Solution
|
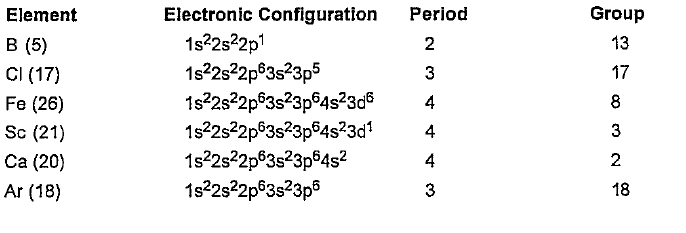
- Give the Electronic configuration and position of the elements in the ...
Text Solution
|
- Why are the cations smaller than atom?
Text Solution
|
- To which block does the element with atomic number 50 belong?
Text Solution
|
- Arrange the increasing order of atomic radii Li, Na, K.
Text Solution
|
- Which is largest among Ca^(+2), Mg^(+2) , Ba^(+2) and why?
Text Solution
|
- Which of the elements Na, Mg, Si and P have the geatest difference bet...
Text Solution
|
- Why Gr-17 elements acts as good oxidising agent?
Text Solution
|
- Arrange Cl, Cl^- , Cl^+ in the increasing order of size?
Text Solution
|
- How does metallic character vary in the group and period?
Text Solution
|
- State the decreasing order of the penetration power of orbitals.
Text Solution
|
- An element has on atomic number = 53. State its position in the Period...
Text Solution
|
- The Reason for diagonal relationship is -
Text Solution
|
- The order of ionic redius of N^(3-), O^(2-), F^- and Na^+ is -
Text Solution
|
- What are actinones?
Text Solution
|
- The elements A, B and C have atomic numbers Z -2, Z and Z +1 respecive...
Text Solution
|
- The elements A, B and C have atomic numbers Z -2, Z and Z +1 respecive...
Text Solution
|
- Why do transition elements have a greater tendency to form co-ordinate...
Text Solution
|
- Give four defects of 'Mendeleev's periodic table.
Text Solution
|