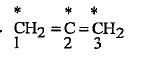Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING-QUESTION BANK
- Give the resonating structures of CO3^(-2)
Text Solution
|
- Give the resonating structures of NO3^(-1)
Text Solution
|
- What is the hybrid state of each carbon overset**CH2=overset**C=overse...
Text Solution
|
- State the hybrid orbital of P in PCl5
Text Solution
|
- State the hybrid orbital of S In SF6.
Text Solution
|
- Calculate the bond order of He2, He2^+.
Text Solution
|
- The number of sigma (sigma) and Pi (pi) bonds present in a molecule of...
Text Solution
|
- How many electrons are involved in bonding in Lewis structure of C2O4^...
Text Solution
|
- Which statement is not correct ?
Text Solution
|
- Which of the following molecules is theoretically not possible and why...
Text Solution
|
- State the hybrid orbital used by Cl in ClO4^(-)
Text Solution
|
- There is no hydrogen bonding in ? epsilon why ?
Text Solution
|
- On the basis of VSEPR theory, discuss the geometry of CH4
Text Solution
|
- On the basis of VSEPR theory, discuss the geometry of NH3 molecule.
Text Solution
|
- Write the molecular orbital Configurations of the following species ...
Text Solution
|
- Write the molecular orbital Configurations of the following species ...
Text Solution
|
- Predict the hybridization state of 'C' in overset |**| marked 'C'
Text Solution
|
- The hybridization of NH3, H2O and CH4 are sp^3 yet the bonds angle of ...
Text Solution
|
- What is the total no of sigma and Pi bonds in the following molecules....
Text Solution
|
- What is the total no of sigma and Pi bonds in the following molecules....
Text Solution
|