Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING-QUESTION BANK
- The observed dipole moment (mu) of HBr molecule is 0.79 D and bond dis...
Text Solution
|
- What is LCAO method ? How does it lead to the concept of bonding and a...
Text Solution
|
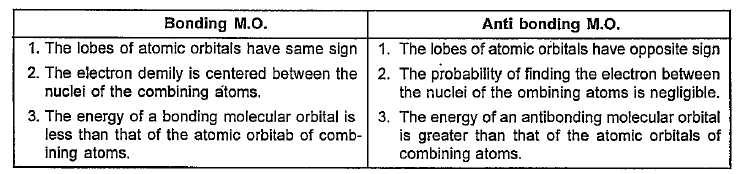
- What are the main points of difference between bonding and anti bondin...
Text Solution
|
- The dipole moment of HCl is 1.03 D If H-Cl bond distance is 1.26 A, ...
Text Solution
|
- If the electronegativity difference between two atoms A and B is 2.0, ...
Text Solution
|
- What is the hybridization of the central atom in ICl2^+ ?
Text Solution
|
- Why is solid NaCl a bad conductor of electricity ?
Text Solution
|
- Explain : CO2 and N2O is polar while CO2 is non-polar ?
Text Solution
|
- Explain : NF3 and NH3 both are tetrahedral, yet NH3 is more polar th...
Text Solution
|
- Explain : NF3 and BF3 both tetra atomic BF3 is nonpolar but NF3 is p...
Text Solution
|
- Explain : HCOOH and C2H6OH having same molecular weight, yet boiling...
Text Solution
|
- Explain : Acidity of maleic acid is greater than fumaric acid.
Text Solution
|
- Account for the following : Ortho nitrophenol is volatile than para ...
Text Solution
|
- Account for the following : BCl3 exist as monomer, but AlCl3 exist a...
Text Solution
|
- Account for the following : HF is least acidic among halogen hydraci...
Text Solution
|
- Explain with reason : SnCl2 is a solid ionic compounds whereas SnCl4 i...
Text Solution
|
- Explain : Melting point of AlF3 is higher than AlCl3
Text Solution
|
- Explain : NCl5 does not exists but PCl5 exists.
Text Solution
|
- The bond angle between two hybrid orbitals is 105^@ . The percentage o...
Text Solution
|
- Write down the types of bonds present in CuSO4 .5H2O.
Text Solution
|