Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-HYDROCARBONS-QUESTION BANK
- Tert - Butylbenzene does not give benzoic acid on treatment with acidi...
Text Solution
|
- Transfer Acetylene to chloroprene.
Text Solution
|
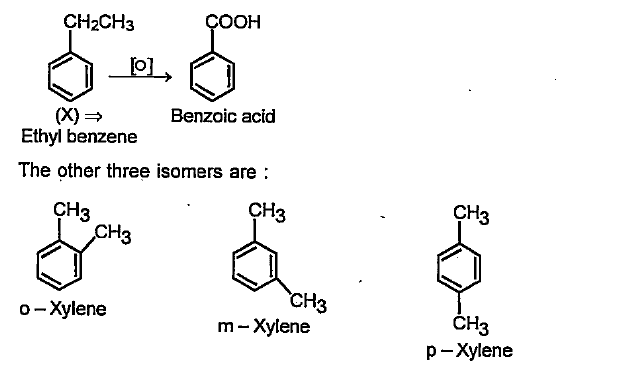
- A hydrocarbon (X) has the molecular formula C8H10. It does not decolou...
Text Solution
|
- Identify A, B and C of the following reaction :
Text Solution
|
Text Solution
|
- How will you account for the formation of ethane during chlorination o...
Text Solution
|
- Write the IUPAC names of the following compounds : CH3CH=C(CH3)2
Text Solution
|
- Write the IUPAC names of the following compounds : CH2=CH-C-=C-CH3
Text Solution
|
- In the alkane CH3-CH2-C(CH3)2-CH2-CH(CH3)2 identify 1^@,2^@,3^@ and 4^...
Text Solution
|
- What is the effect of branching of alkane chain on its boiling point?
Text Solution
|
- Why Wurtz reaction cannot be used to prepare pure alkanes with odd num...
Text Solution
|
- find the product of acid catalysed dehydration
Text Solution
|
Text Solution
|
- What happens when 1,3 butadiene reacts with maleic anhydride?
Text Solution
|
- Write the IUPAC names of the products obtained by thr ozonolysis of th...
Text Solution
|
- Write the IUPAC names of the products obtained by thr ozonolysis of th...
Text Solution
|
- Write the IUPAC names of the products obtained by the ozonolysis of th...
Text Solution
|
- Write the IUPAC names of the products obtained by thr ozonolysis of th...
Text Solution
|
- An alkene 'A' upon ozonolysis gives a mixture of ethanal and pentan-3-...
Text Solution
|
- An alkene 'A' contains three C-C eight C-H and one C-C(pi) bonds upon ...
Text Solution
|