Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-EQUILIBRIUM-QUESTION BANK
- Write the conjugate acids of CN^-,Cl^-,Cl^-,H^-,HSO3^-. "The conjugate...
Text Solution
|
- Write the differences between : Solubility and solubility product
Text Solution
|
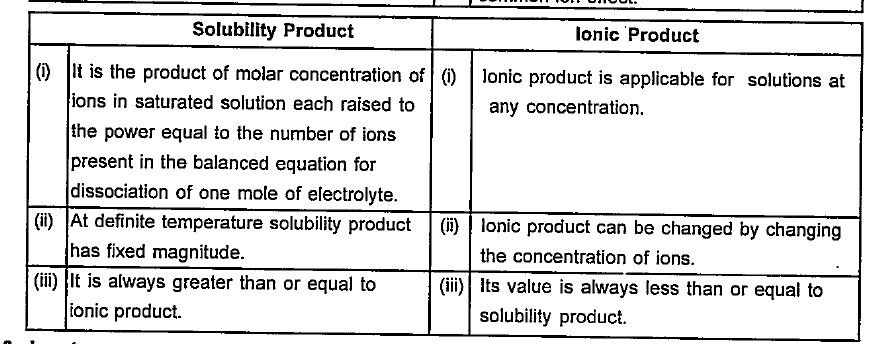
- Write the differences between : Solubility product and ionic product...
Text Solution
|
- Whether the pH of aqueous solution of ammonium chloride is more or les...
Text Solution
|
- Write the Ostwald Dilution law. What are its limitations? Deduce the m...
Text Solution
|
- What is common ion effect? Give example. What is buffer action?
Text Solution
|
- Explain why: Solubility of magnesium hydroxide increases in ammonium...
Text Solution
|
- Explain why: Solubility of PbCl2 decreases on addition of KCl soluti...
Text Solution
|
- What is an isohydric solution?
Text Solution
|
- Explain what happens when H2S gas is passed through acidified mixtur...
Text Solution
|
- Explain what happens when HCl gas is passed through saturated soluti...
Text Solution
|
- Explain buffer capacity of a buffer solution.
Text Solution
|
- An acid of pH 5 is diluted 1000 times. What is the pH of final solutio...
Text Solution
|
- An acid of pH 5 is diluted 1000 times. What is the pH of final solutio...
Text Solution
|
- The correct order of increasing basicily of the given conjugate bases ...
Text Solution
|
- Can methyl orange indicator be used in the titration of 0.1 M acetic a...
Text Solution
|
- Determine the pH of a solution which is obtained by mixing equal volum...
Text Solution
|
- Determine the pH of 10^(-8) M HCl acid solution. Show that, degree of ...
Text Solution
|
- The solubility product of Mn(OH)2 at 25^@C is 1.9xx10^(-13). What is t...
Text Solution
|
- The pH of a buffer solution remains almost same on dilution - explain.
Text Solution
|