Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-EQUILIBRIUM-QUESTION BANK
- The equilibrium constant for the reaction N2(g) +O2(g)iff2NO is K1 ...
Text Solution
|
- What happens when Arsenic oxide reacts with hydrogen sulphide?
Text Solution
|
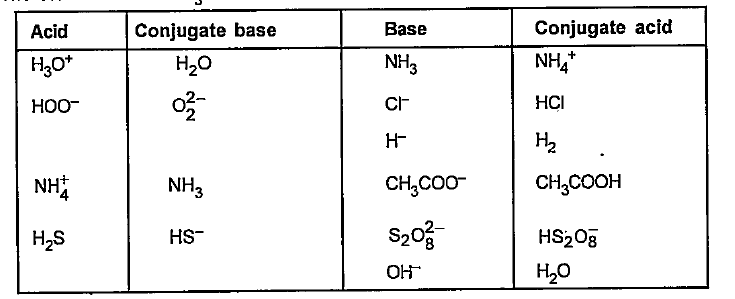
- Classifty the following as acid and base according ot Bronsted and Low...
Text Solution
|
- If 50 ml 0.2 (M) CH3COOH is mixed with 20 ml 0.2 (M) NaOH solution, th...
Text Solution
|
- At a given temperature, if the solubility product and solubility of a ...
Text Solution
|
- Explain why pH of 0.1 (M) CH3COOH solution is higher than that of 0.1 ...
Text Solution
|
- Which of the given salts will undergo cationic or anionic or both cati...
Text Solution
|
- At a ertain temperature, the ration of ionisation constants of a weak ...
Text Solution
|
- What would be nature of aqueous solutions of NaF and NH4NO3.
Text Solution
|
- In aqueous solution, a weak tribasic acid H3A ionises in the following...
Text Solution
|
- At 46^@C, Kp for the reaction N2O4(g) iff 2NO2(g) is 0.66. Calculate t...
Text Solution
|
- Ca(HCO3)2 decomposes as Ca(HCO3)2(s)→CaCO3 (s)+H2O (g)+CO2 (g). Total ...
Text Solution
|
- alpha- D glucose undergoes mutarotation to beta- D glucose in aqueous ...
Text Solution
|
- The rate of the elementary gaseous phase reaction. 2NO+O2 iff 2NO2 a...
Text Solution
|
- The following concentrations were obtained for the formation of NH3 fr...
Text Solution
|
- At equilibrium, the concentrations of N2 = 3.0 xx 10^-3M, O2 - 4.2 xx ...
Text Solution
|
- The value of Kc = 4.24 at 800K for the reaction, CO(g) + H2O(g) iff C...
Text Solution
|
- Equilibrium constants are given (in atm) for the following reactions a...
Text Solution
|
- Equilibrium constants are given (in atm) for the following reactions a...
Text Solution
|
- The value of Kc for the reaction 2A iff B+C is 2 xx 10^-3, At a given...
Text Solution
|