Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-SOME P-BLOCK ELEMENTS-QUESTION BANK
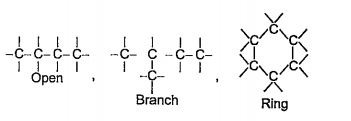
- How carbon differs from other elements of group 14?
Text Solution
|
- Dimond is non conductor of electricity but Graphatite is a conductor o...
Text Solution
|
- Why carbon exhibits catenation character more than other elements of g...
Text Solution
|
- Write the resonating structure for CO3^(2-) and HCO3^-.
Text Solution
|
- What are Fullerenes?
Text Solution
|
- State what happens when orthoboric acid is heated gradually.
Text Solution
|
- Carbon monoxide has reducing property while carbon dioxide acts as oxi...
Text Solution
|
- Why is carbone monoxide toxic?
Text Solution
|
- How can you get pure CO from potassium ferrocyanide?
Text Solution
|
- Why is dilute H2SO4 not used in the Laboratory preparation of CO2?
Text Solution
|
- How can you separate CO from a mixture of CO and CO2?
Text Solution
|
- Why SiCl4 behaves as a Lewis acid?
Text Solution
|
- What are Zeolites?
Text Solution
|
- What is tetra chloro silico methane? How it can be prepared from Silic...
Text Solution
|
- What are Silicones? Why are they so called?
Text Solution
|
- What are silicates?
Text Solution
|
- Explains why CO2 is gaseous while SiO2 is a Solid?
Text Solution
|
- Write two uses of Silicones.
Text Solution
|
- Why BF6^(3-) does not exist, explain.
Text Solution
|
- White fumes appear when bottles containing anhydrous AlCl3 is opened f...
Text Solution
|