Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-SOME P-BLOCK ELEMENTS-QUESTION BANK
- Explain: Silicon forms Si F6^(2-) but corresponding fluoro compound ...
Text Solution
|
- Explain: BCl3overset("HOH")rarr[B(OH)4] but AlCl3overset("HOH")under...
Text Solution
|
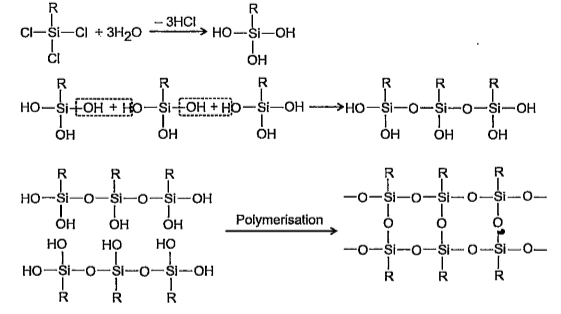
- If the starting material for manufacture of silicones is RSiCl3 then w...
Text Solution
|
- What is inorganic graphite? Why is it so called?
Text Solution
|
- Why B-F bond lengths in BF3 and BF^-4 differ ?
Text Solution
|
- PbCl2 is more stable than PbCl4 - Why?
Text Solution
|
- Trisillylamine is a weaker base than trimethylamine-Why?
Text Solution
|
- Out of CO and CO2 which acts as an ligand and why?
Text Solution
|
- What do you mean by oil dag and aqua dag ? Write their uses.
Text Solution
|
- What happens when (State with equation) At 200^@C under high pressur...
Text Solution
|
- What happens when Silicon is heated with methyl chloride at high tempe...
Text Solution
|
- Identify the compound A, X and Z in the following. A+2HCl+5H2O to 2N...
Text Solution
|
- Identify the compound A, X and Z in the following.
Text Solution
|
- Complete the following: ---+3LiAiH4 to ---+3LiF+3AiF3
Text Solution
|
- Complete the following: ---+6H2O to---+6H2
Text Solution
|
- Complete the following: ---+3O2 overset (triangle) to B2O3+3H2O
Text Solution
|
- Describe the shapes of BF3 and BF4^
Text Solution
|
- Is boric acid a protonic acid ? Explain.
Text Solution
|
- What is importance of highly pure silicon? How can it be prepared?
Text Solution
|
- Alkanes are large in number but Sillanes are comparatively few- Why?
Text Solution
|