Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-SOME P-BLOCK ELEMENTS-QUESTION BANK
- Complete the following: ---+6H2O to---+6H2
Text Solution
|
- Complete the following: ---+3O2 overset (triangle) to B2O3+3H2O
Text Solution
|
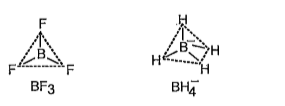
- Describe the shapes of BF3 and BF4^
Text Solution
|
- Is boric acid a protonic acid ? Explain.
Text Solution
|
- What is importance of highly pure silicon? How can it be prepared?
Text Solution
|
- Alkanes are large in number but Sillanes are comparatively few- Why?
Text Solution
|
- What are silicates? Give one example each of orthosilicate and pyrosil...
Text Solution
|
- What happens when (Give equation) Calcium carbonate is strongly heat...
Text Solution
|
- What happens when (Give equation) Carbon monoxide gas is passed oven...
Text Solution
|
- Why is the CO2 molecule non polar?
Text Solution
|
- Write down the condition and equation of the reaction between ammonia ...
Text Solution
|
- A mixture of CO and CO2 gas is there. How would you convert the whole ...
Text Solution
|
- A mixture of CO and CO2 gas is there. How would you convert the whole ...
Text Solution
|
- Which compound on heating gives pure BF3?
Text Solution
|
- What are Carbogen and smelling salt? Give one use of each of them.
Text Solution
|
- Between AIF3 and AlCl3 which has greater melting point?
Text Solution
|
- Anhydrous AlCl3 is a covalent compound but hydrated aluminium chloride...
Text Solution
|
- PbCl4 is a stable compound but Pbl4 has no existance. Explain Why?
Text Solution
|
- What is tynchal? Give its chemical formula.
Text Solution
|
- How would you remove CO2 from a mixture of CO2 and SO2
Text Solution
|