Similar Questions
Explore conceptually related problems
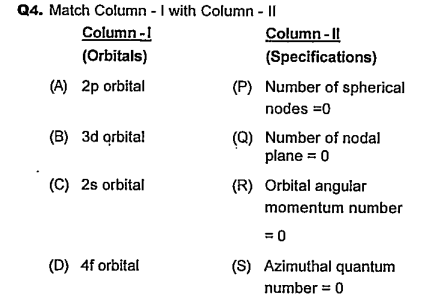
PATHFINDER-ATOMIC STRUCTURE-QUESTION BANK
- Match column I with column II
Text Solution
|
- Match column I with column II
Text Solution
|
- Match column I with column II
Text Solution
|
- Match column I with column II
Text Solution
|
- An oil drop has 8.01xx10^-19C charge calculate the number of electrons...
Text Solution
|
- The wavelength of m^(th) line in Balmer series for an orbitals is 4103...
Text Solution
|
- The velocity of an electron in a certain Bohr's orbit of H atom bears ...
Text Solution
|
- An ion Mn^(a+) has the magnetic moment equals to 4.9 BM what is the va...
Text Solution
|
- The number of waves made by a Bohr electron in an orbit of maximum mag...
Text Solution
|
- Iodine molecule dissociates into atoms after absorbing light of 4500A^...
Text Solution
|
- After the collision of two H atoms each atom emits a photon of wavelen...
Text Solution
|
- Find the quantum number 'n' corresponding to the excited state of He^+...
Text Solution
|
- What is highest frequency of the photon that can be emitted from H ato...
Text Solution
|
- Calculate the longest wavelength for the transition in the Paschen ser...
Text Solution
|
- Calculate the ratio of the wavelength of first and the ultimate line o...
Text Solution
|
- What hydrogen like ion has wavelength difference between the first lin...
Text Solution
|
- The wavelength corresponding to a transition when electron falls from ...
Text Solution
|
- When certain metal was irradiated with light of frequency 1.6xx10^16Hz...
Text Solution
|
- Assume that 2xx10^(-17)J of light energy is needed by the interior of ...
Text Solution
|
- What is the energy content per photon (J) for light of frequency 4.2xx...
Text Solution
|