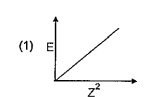
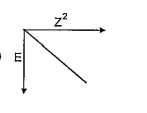
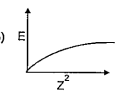
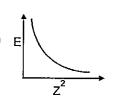
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-ATOMIC STRUCTURE-QUESTION BANK
- If the total energy of an electron in the ist shell of H-atom =-13.6eV...
Text Solution
|
- What is the potential energy of an electron present in N-shell of the ...
Text Solution
|
- The energy of an electron moving in nth bohr's orbit of an element is ...
Text Solution
|
- The energy of the electron in the hydrogen atom is given by the expres...
Text Solution
|
- The ratio of the energy of the electron in ground state of H to the el...
Text Solution
|
- The ratio (E2-E1) to (E4-E3) for the hydrogen atom is approximately eq...
Text Solution
|
- If the ionization energy of Li^(+2) is 19.6xx10^(-18) J per atom then ...
Text Solution
|
- The spectrum produced from an element is
Text Solution
|
- The wave number of first line of Balmer series of hydrogen is 15200 cm...
Text Solution
|
- Find the value of wave number In terms of Rydberg's constant , when tr...
Text Solution
|
- The radiation emitted when a hydrogen atom goes from a higher energy s...
Text Solution
|
- H- atoms in ground state (13.6eV) are excited by monochromatic radiati...
Text Solution
|
- A hydrogen atom in the ground state is excited by monochromatic radiat...
Text Solution
|
- For silver metal the thershold frequency nu0 is 1.13xx10^(17) Hz what ...
Text Solution
|
- The number of elliptical orbits excluding circular orbits in the N-she...
Text Solution
|
- The wavelength associated with a golf ball weighing 200g and moving at...
Text Solution
|
- What should be velocity of an electron so that its momentum becomes eq...
Text Solution
|
- The momentum of photon having 6Mev energy is
Text Solution
|
- If the radius of first Bohr orbit of H atom is x then de Broglie wavel...
Text Solution
|
- Write the number of waves made by an electron moving in an orbit havin...
Text Solution
|