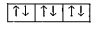
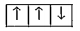
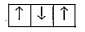
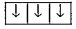
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-ATOMIC STRUCTURE-QUESTION BANK
- The french physicist Louis de Broglie in 1924 postulated that matter l...
Text Solution
|
- Spin angular momentum of an electron has no analog in classical mechan...
Text Solution
|
- Spin angular momentum of an electron has no analog in classical mechan...
Text Solution
|
- Match column -i with Column -ii
Text Solution
|
- Match column -i with Column -ii
Text Solution
|
- Match column -i with Column -ii
Text Solution
|
- Match column -i with Column -ii
Text Solution
|
- Match column -i with Column -ii
Text Solution
|
- In the Bohr orbits if the mass of electron is halved, the radius will ...
Text Solution
|
- What is the ratio of wavelength of ii line of balmer series and i line...
Text Solution
|
- H- atoms in ground state (13.6eV) are excited by monochromatic radiati...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|
- Out of the following in how many pairs energy sequence is reversed aft...
Text Solution
|