A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTION BANK
- Screening effect is the effect produced by intervening electrons betwe...
Text Solution
|
- Screening effect is the effect produced by intervening electrons betwe...
Text Solution
|
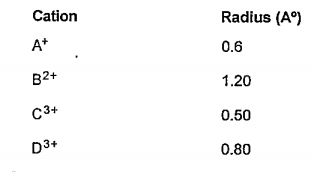
- You have given cations A^+ , B^(2+) , C^(3+) D^(3+) The radius of the...
Text Solution
|
- You have given cations A^+ , B^(2+) , C^(3+) D^(3+) The radius of the...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- IE and EA values of an element are 13eV and 3.8 eV respectively. Its e...
Text Solution
|
- How many unpaired electron are present Co^(3+) ion?
Text Solution
|
- The effective nuclear charge of N atom is :
Text Solution
|
- The ionisation energy of lithium is 500 KJ mol^(-1) The amount of ener...
Text Solution
|
- How many groups are occupied by P-block element in the long form of pe...
Text Solution
|
- Why the electron gain enthalpy values of alkaline earth metals are low...
Text Solution
|
- Select neutral acidic basic and amphoteric oxides from the following ...
Text Solution
|
- Ionisation energy and electron affinity of fluorine are respectively 1...
Text Solution
|
- Match the following columns
Text Solution
|
- Arrange the following ions in the increasing order of their size :Be^(...
Text Solution
|
- Match the following columns
Text Solution
|