Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTION BANK
- Arrange the following ions in the increasing order of their size :Be^(...
Text Solution
|
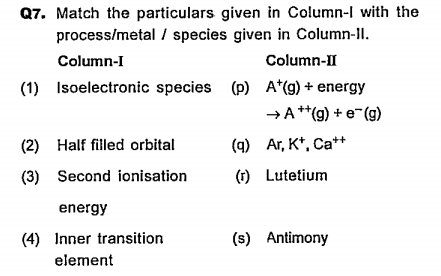
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- As per the modern periodic law the physical and chemical properties of...
Text Solution
|
- Which of these does not reflect the periodicity of the elements
Text Solution
|
- Which of the following pairs of atomic numbers represent elements belo...
Text Solution
|
- All the elements in a group in the periodic table have the same
Text Solution
|
- The elements in which 4f orbitals are progressively filled up are call...
Text Solution
|
- Which of the following elements does not belong to first transition se...
Text Solution
|
- The name of 'rare earths' is used for
Text Solution
|
- In genral outer electronic configuration of the elements of group ViB ...
Text Solution
|
- What is the group no.of an element having atomic no.105?
Text Solution
|
- Which of the following ionic radius would be maximum?
Text Solution
|
- The decreasing order of size of the following ions is
Text Solution
|
- Na+,Mg^(++),Al^(+3),Si^(+4) are isoelectronics the order of their ioni...
Text Solution
|
- The covalent and van der waals radii of hydrogen respectively are
Text Solution
|
- which ion possessses the smallest radius?
Text Solution
|
- Which of the following isoelectronic ions has lowest ionization energy...
Text Solution
|
- The first ionization potential in electron volts of nitrogen and oxyge...
Text Solution
|