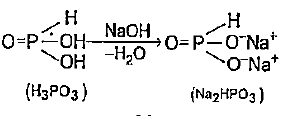Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-MOLE AND STOICHIOMETRY-I-QUESTION BANK
- Determine the number of gram equivalents of solute in 200 mL of 0.1 ...
Text Solution
|
- Calculate equivalent mass (E) of the following - (1) K-Alum (molar m...
Text Solution
|
- Calculate equivalent mass (E) of the following - H3PO3 (molar mass = ...
Text Solution
|
- Specific heat of a metal is 0.031 cal per gram, and its eq. wt is 103....
Text Solution
|
- A chloride of an element contain 49.5% chlorine. The specific heat of ...
Text Solution
|
- On dissolving 2.0 g of metal in sulphuric acid, 4.51 g of the metal su...
Text Solution
|
- How are 0.50 mol Na2CO3 and 0.50 M Na2CO3 different ?
Text Solution
|
- Potassium sulphate (S = 18.38%)and potassium selenate Se = 35.75%) are...
Text Solution
|
- If the density of methanol is 0.793 kg L^(-1), what is its volume need...
Text Solution
|
- Carbon combines with hydrogen to form three compounds A, B and C. The ...
Text Solution
|
- Aluminium oxide contains 53% Al and carbon dioxide contains 28% C. Ass...
Text Solution
|
- Calculate the percentage composition of calcium nitrate.
Text Solution
|
- In the reaction A + B2 rarr AB2, Identify the limiting reagent, if any...
Text Solution
|
- In the reaction A + B2 rarr AB2, Identify the limiting reagent, if any...
Text Solution
|
- Dinitrogen and dihydrogen react with each other to produce ammonia acc...
Text Solution
|
- Dinitrogen and dihydrogen react with each other to produce ammonia acc...
Text Solution
|
- Dinitrogen and dihydrogen react with each other to produce ammonia acc...
Text Solution
|
- A compound contains 4.07% hydrogen, 24.27% carbon and 71.65% chlorine ...
Text Solution
|
- Draw the structure of Vanilin.
Text Solution
|
- How many moles of electrons weigh one kilogram ?
Text Solution
|