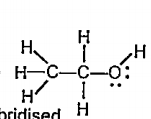
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Which hybrid orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- The correct order of the bond angles is
Text Solution
|
- Which of the following has pyramidal shape ?
Text Solution
|
- The shape of methyl carbocation (CH3^+) is likely to be :
Text Solution
|
- Bond length of which of the following types of bonds is maximum ?
Text Solution
|
- In the XeF4 molecule, the Xe atom is in the
Text Solution
|
- The shape of IF5 and IF7 are respectively
Text Solution
|
- The shape of a molecule which has 3 bond pairs and one lone pair is
Text Solution
|
- Although geometries of NH3 and H2O molecules are distorted tetrahedral...
Text Solution
|
- The melting point of KCl is higher than that of AgCl though the crysta...
Text Solution
|
- SnF4 has boiling point 705^@C while SnCl4 has 114^@C -explain
Text Solution
|
- The solubility of silver halides in water decreases from AgF to AgI. E...
Text Solution
|
- Calculate the % of ionic character of a bond having bond length=0.92ov...
Text Solution
|
- Which of the following have zero dipolemoment
Text Solution
|
- Dipole moment of HCl is 1.03 D and its bond length is 1.257 overset@A....
Text Solution
|
- Certain bond has 76.81% ionic character. If the bond length is 159.6 p...
Text Solution
|