Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Which of the following is not correct ?
Text Solution
|
- Write the significance/applications of dipole moment ?
Text Solution
|
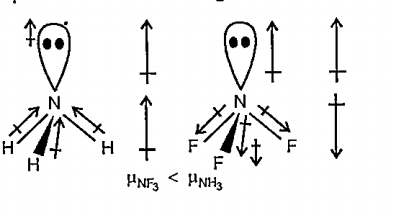
- Which out of NH3 and NF3 has higher dipole moment and why ?
Text Solution
|
- Which of the following compounds contain ionic, covalent, coordinate c...
Text Solution
|
- Which of the following compounds would have significant intermolecular...
Text Solution
|
- Which one of the following does not have intermolecular H-bonding ?
Text Solution
|
- Ethanol has a higher boiling point than dimethyl ether through they ha...
Text Solution
|
- Explain the important aspects of resonance to the CO3^(2-) ion.
Text Solution
|
- Write the resonance structures for NO2
Text Solution
|
- Write the resonance structures for NO3^-
Text Solution
|
- Compare the bond length O-O in following molecules: KO2, O2, O2 [AsF6]
Text Solution
|
- Which of the following species has bond order of zero
Text Solution
|
- Use molecular orbital theory to explain why the Be2 molecule does not ...
Text Solution
|
- Compare the relative stability of the following species and indicate t...
Text Solution
|
- Among the following isostructural compounds, the compound, with highes...
Text Solution
|
- An electrovalent compound does not exhibit space isomerism because of
Text Solution
|
- The correct increasing order of Ionic character among the following co...
Text Solution
|
- The pair of elements which on combination are most likely to form an i...
Text Solution
|
- Sodium sulphate is soluble in water but barium sulphate is sparingly s...
Text Solution
|
- The types of bond present in N2O5 vapour state
Text Solution
|