Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Ethanol has a higher boiling point than dimethyl ether through they ha...
Text Solution
|
- Explain the important aspects of resonance to the CO3^(2-) ion.
Text Solution
|
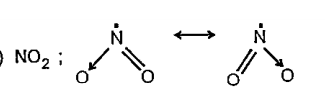
- Write the resonance structures for NO2
Text Solution
|
- Write the resonance structures for NO3^-
Text Solution
|
- Compare the bond length O-O in following molecules: KO2, O2, O2 [AsF6]
Text Solution
|
- Which of the following species has bond order of zero
Text Solution
|
- Use molecular orbital theory to explain why the Be2 molecule does not ...
Text Solution
|
- Compare the relative stability of the following species and indicate t...
Text Solution
|
- Among the following isostructural compounds, the compound, with highes...
Text Solution
|
- An electrovalent compound does not exhibit space isomerism because of
Text Solution
|
- The correct increasing order of Ionic character among the following co...
Text Solution
|
- The pair of elements which on combination are most likely to form an i...
Text Solution
|
- Sodium sulphate is soluble in water but barium sulphate is sparingly s...
Text Solution
|
- The types of bond present in N2O5 vapour state
Text Solution
|
- In which of the following compounds, breaking of covalent bond takes p...
Text Solution
|
- The valency of sulphur in sulphuric acid is
Text Solution
|
- when 2s-2s,2p-2p and 2p-2s orbitals overlap, the bond strength decreas...
Text Solution
|
- The ratio of sigma and pi bonds in benzene is:
Text Solution
|
- Indicate the wrong statement
Text Solution
|
- Which of the following molecules does not have coordinate covalent bon...
Text Solution
|