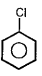
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Which of the following has lowest melting point?
Text Solution
|
- The experimental value of the dipole moment of HCl is 1.03D. The lengt...
Text Solution
|
- The dipole moment of is
Text Solution
|
- Which of the following exhibit H-bonding ?
Text Solution
|
- The volatility of HF is low because of:
Text Solution
|
- Intramolecular hydrogen bonding is found in
Text Solution
|
- Among liq. NH3, liq. HF, CH4, CH3OH and N2O4 intermolecular hydrogen b...
Text Solution
|
- Two ice cubes are pressed over each other and unite to form one cube. ...
Text Solution
|
- Nitrous oxide has dipole moment
Text Solution
|
- What is the formal charge on sulphur in H2SO4?
Text Solution
|
- Out of the following four resonance structures for the CO2 molecules, ...
Text Solution
|
- Which among the following structures can not respresent resonance form...
Text Solution
|
- Regarding the most stable resonating form which of the following is co...
Text Solution
|
- Which of the following order of energies of molecular orbitals of N2 i...
Text Solution
|
- Which of the following statements is not correct from the view point o...
Text Solution
|
- Which of the following options represents the correct bond order?
Text Solution
|
- Main axos of a diatomic molecule is Z orbitals Px and Py of two atoms ...
Text Solution
|
- In a molecule of ethyne, the number of pi antibondding molecular orbit...
Text Solution
|
- In diborane (B2H6) the stability of B-H-B (3c-2e) bond can be explaine...
Text Solution
|
- Which of the following combination will give the strongest ionic bond ...
Text Solution
|