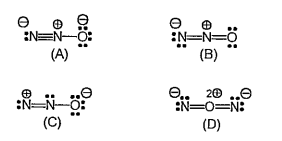
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- What is the formal charge on sulphur in H2SO4?
Text Solution
|
- Out of the following four resonance structures for the CO2 molecules, ...
Text Solution
|
- Which among the following structures can not respresent resonance form...
Text Solution
|
- Regarding the most stable resonating form which of the following is co...
Text Solution
|
- Which of the following order of energies of molecular orbitals of N2 i...
Text Solution
|
- Which of the following statements is not correct from the view point o...
Text Solution
|
- Which of the following options represents the correct bond order?
Text Solution
|
- Main axos of a diatomic molecule is Z orbitals Px and Py of two atoms ...
Text Solution
|
- In a molecule of ethyne, the number of pi antibondding molecular orbit...
Text Solution
|
- In diborane (B2H6) the stability of B-H-B (3c-2e) bond can be explaine...
Text Solution
|
- Which of the following combination will give the strongest ionic bond ...
Text Solution
|
- In NO3^- ion, the number of bond pairs and lone pairs of electrons on ...
Text Solution
|
- Number of sigma-bonds and pi-bonds in the following structure is
Text Solution
|
- In which of the following molecules/ions all the bonds are not equal ?
Text Solution
|
- If the electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^...
Text Solution
|
- Stable form of A may be represented by formula [A is a member of inert...
Text Solution
|
- Stable form of C may be represented by formula [C is a member of halog...
Text Solution
|
- The molecular formula of the compound formed between B and C will be [...
Text Solution
|
- The bond between B and C will be [B and C are the member of group 15 a...
Text Solution
|
- Which of the following would result in the formation of strongest pi-b...
Text Solution
|