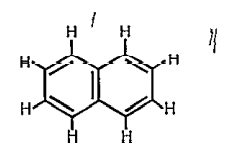
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Which of the following combination will give the strongest ionic bond ...
Text Solution
|
- In NO3^- ion, the number of bond pairs and lone pairs of electrons on ...
Text Solution
|
- Number of sigma-bonds and pi-bonds in the following structure is
Text Solution
|
- In which of the following molecules/ions all the bonds are not equal ?
Text Solution
|
- If the electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^...
Text Solution
|
- Stable form of A may be represented by formula [A is a member of inert...
Text Solution
|
- Stable form of C may be represented by formula [C is a member of halog...
Text Solution
|
- The molecular formula of the compound formed between B and C will be [...
Text Solution
|
- The bond between B and C will be [B and C are the member of group 15 a...
Text Solution
|
- Which of the following would result in the formation of strongest pi-b...
Text Solution
|
- The type of d-orbital involved in sp^3d hybridisation is
Text Solution
|
- In which of the following there is change in the type of hybridization...
Text Solution
|
- If MX3 is T shaped, then the number of lone pair around M is
Text Solution
|
- Which of the following species have same shape and same bond order ?
Text Solution
|
- Isostructural species are those which have the same shape and hybridis...
Text Solution
|
- The types of hybrid orbitals of nitrogen in NO2^+ NO3^- and NH4^+ resp...
Text Solution
|
- Which of the following species has tetrahedral geometry ?
Text Solution
|
- Which of the following angle corresponds to sp^2 hybridisation ?
Text Solution
|
- Maximum change in percentage of s-character of bonding orbital of cent...
Text Solution
|
- The correct decreasing order of nearest bond angle:
Text Solution
|