Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Arrange the following in the order of increasing NO2^+,NO2^-, NO3^- i...
Text Solution
|
- Arrange the following in the order of increasing NO, NO^+ and NO^- in...
Text Solution
|
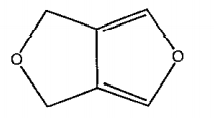
- Consider the following structure Find the total number of hybridised...
Text Solution
|
- Find the total number of pi electrons in the following compound.
Text Solution
|
- In methanoic acid, one carbon oxygen bond length is 123 pm and another...
Text Solution
|
- Ammonium salts are much more soluble in water than the corresponding s...
Text Solution
|
- Amongst o-hydroxybenzalaldehyde and p-hydroxybenzaldehyde which is mo...
Text Solution
|
- o-nitrophenol is steam volatile but p nitrophenol is not. Explain.
Text Solution
|
- In which of the following solvents, KI has highest solubility ? The di...
Text Solution
|
- Which is the most ionic ?
Text Solution
|
- The correct order of the increasing ionic character is
Text Solution
|
- Which of the following pair of elements form a compound with maximum i...
Text Solution
|
- ppi-dpi back bonding occurs between oxygen and
Text Solution
|
- What is the no. of sigma and pi bonds presents in a molecule of H2SO4 ...
Text Solution
|
- The molecule which contain ionic, covalent and co-ordinate bond is
Text Solution
|
- Which of the following halides has different bond lengths ?
Text Solution
|
- Which of the following is isostructural with NH3 ?
Text Solution
|
- H-B-H bond angle in BH4^- is
Text Solution
|
- The pair of species having identical shape is
Text Solution
|
- Structure of ICl2^- is
Text Solution
|