Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
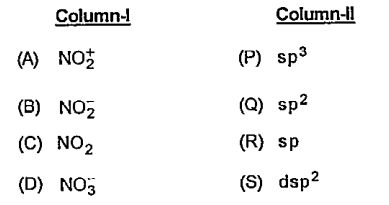
- Match the column-I (ions) with Column-II (shapes) and select the corre...
Text Solution
|
- Match the column-I (compound) with Column-II (molecular shape) and sel...
Text Solution
|
- Match the column-I (species) with Column-II (type of hybridisation) an...
Text Solution
|
- https://d10lpgp6xz60nq.cloudfront.net/physicsimages/PATCHE0XIB05C11E01...
Text Solution
|
- The number of dpi-ppi bonding in SO3 molecule
Text Solution
|
- The number of P-P bond in P4O10 is
Text Solution
|
- The number of hybrid orbitals at 120^@ in BF3 is
Text Solution
|
- The number of water molecules that are directly bonded with Fe in FeSO...
Text Solution
|
- The number of lone pair on central atom in ClF3
Text Solution
|
- Draw the molecular structures of XeF2, XeF4 and XeO2F2 indicating the ...
Text Solution
|
- Using VSEPR theory, identify the type of hybridisation and draw the ...
Text Solution
|
- In SF4 molecule, the lone pair of electrons occupy equatorial position...
Text Solution
|
- Apart from tetrahedral geometry, another possible geometry for CH4 is ...
Text Solution
|
- Arrange the bonds in the order of increasing ionic character in the mo...
Text Solution
|
- Calculate the % ionic character in HCI molecule. Given bond length of ...
Text Solution
|
- The dipole moment of LiH is 1.964xx10^(-29) Cm and the intermolecular ...
Text Solution
|
- A diatomic molecule has a dipole moment equal to 1.2.D. If bond distan...
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
- The number of lone pair of electrons on the central atoms of H2O, SnCl...
Text Solution
|
- In the following compound, the number of 'sp' hybridisation carbon is ...
Text Solution
|