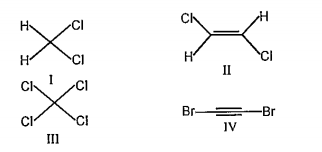
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- The structure of XeF6 is experimentally determined to be distorted oct...
Text Solution
|
- Which one of the following species has plane triangular shape ?
Text Solution
|
- The compound that will have a permanent dipole moment among the follow...
Text Solution
|
- The correct order of decreasing length of the bond as indicated by the...
Text Solution
|
- The pair of compounds having identical shapes for their molecules is
Text Solution
|
- The correct arrangement of the species in the decreasing order of the ...
Text Solution
|
- Which of the following is the correct order for strength of C-X bond ?
Text Solution
|
- What is the geometry of molecule of bromine penta fluoride ?
Text Solution
|
- Bond order of which among the following molecules is zero ?
Text Solution
|
- The bond order in the superoxide ion (O2^-) is
Text Solution
|
- The ion which is not tetrahedral in shape is
Text Solution
|
- Dipole-induced dipole interactions are present in which of the followi...
Text Solution
|
- Which one of the following molecules contain no pi-bond ?
Text Solution
|
- Which one of the following is paramagnetic?
Text Solution
|
- Geometry of SF2Cl2 molecule is
Text Solution
|
- Which of the following represents the given mode of hybridisation sp^2...
Text Solution
|
- Which of the following is least stable ?
Text Solution
|
- Which of the following will have the maximum dipole moment
Text Solution
|
- The volume of oxygen liberated 0.68 g of H2O2 is
Text Solution
|
- The number of pi-bond in CH2=CH-CH=CH-C-=CH is
Text Solution
|