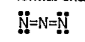A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Which of the following is not tetrahedral ?
Text Solution
|
- During the formation of a chemical bond
Text Solution
|
- In the following electron-dot structure, calculate the formal charge f...
Text Solution
|
- Hybridisation of C2 and C3 of H3C-C((2)) H=C((3))=CH-CH3 are
Text Solution
|
- Which of the following Vitamin deficiency causes Beri-Beri?
Text Solution
|
- Which one of the following is paramagnetic?
Text Solution
|
- Among the following the maximum covalent character is shown by the com...
Text Solution
|
- The hybridisation of orbitals of N atom in NO3^(-), NO2^(+) and NH4^(+...
Text Solution
|
- The structure of IF7 is
Text Solution
|
- Which of the following has maximum number of lone pairs associated wi...
Text Solution
|
- The number of types of bonds between two carbon atoms in calcium carbi...
Text Solution
|
- Number of non-bonding electron pair on Xe in XeF6, XeF4 and XeF2 respe...
Text Solution
|
- The number of sigma (sigma) and pi (pi) covalent bonds respectively in...
Text Solution
|
- Hybridisation shown by carbon and oxygen of -OH group in phenol are re...
Text Solution
|
- Intramolecular hydrogen bond is present in water o-nitrophenol p-nit...
Text Solution
|
- Which of the following is not correct with respect to bond order of th...
Text Solution
|
- When O2 is converted into O2^(+)
Text Solution
|
- The correct order of increasing bond length of C-H, C-O, C-C and C=C i...
Text Solution
|
- Considering the state of hybridisation of carbon atoms, find out the m...
Text Solution
|
- Which of the two ions from the list given below that have the geometry...
Text Solution
|