A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- A 2 g sample of hydrogen at temperature T and of volume V exerts press...
Text Solution
|
- The vapour pressure of water is 17.5 torr at 20^@C. When the relative ...
Text Solution
|
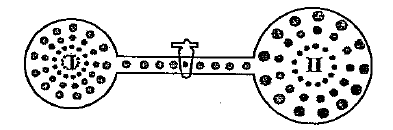
- Two bulbs are connected with a tube of negligible volume. Bulb I ...
Text Solution
|
- A vessel of unknown volume V contains a mixture of propane and methane...
Text Solution
|
- What is the pressure of 2 mole of NH3at27^@C when its volume is 5 litr...
Text Solution
|
- If the mean free path is 'a' at one atm pressure, then its value at 5 ...
Text Solution
|
- The relationship between Pc,Vc and Tc is
Text Solution
|
- a' and 'b' are van der Waals constants for gases. Chlorine is more eas...
Text Solution
|
- What happens when ammonia reacts with excess of chlorine?
Text Solution
|
- The critical temperature and reduced temperature of a gas are 150 K an...
Text Solution
|
- In terms of critical constants, the compressibility factor is
Text Solution
|
- Which of the following statements is correct about a liquid at constan...
Text Solution
|
- The integrated form of Clausius-Clapeyron equation is:
Text Solution
|
- The surface tension of which of the following liquid is maximum ?
Text Solution
|
- If eta is the coefficient of viscosity and T is the temperature in kel...
Text Solution
|
- The boiling points of water, ethyl alcohol and diethyl ether are 100^@...
Text Solution
|
- The circulation of blood in human body supplies O2 and releases CO2, t...
Text Solution
|
- A mixture of NH3 (g) and N2H4 (g) is placed in a sealed container at 3...
Text Solution
|
- What happens when excess ammonia reacts with chlorine?
Text Solution
|
- A box is divided into two equal parts A and B having two gases X and Y...
Text Solution
|