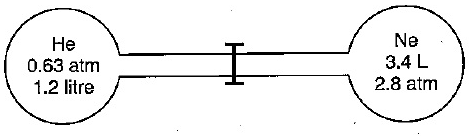
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- Ten moles of an ideal gas are filled In a closed vessel. The vessel ha...
Text Solution
|
- A flask filled with C Cl4 vapour was weighed at a temperature and pres...
Text Solution
|
- Two gas bulbs are connected by a thin tube. Calculate the partial pres...
Text Solution
|
- A large cylinder of helium filled at 2000 mm of Hg had a small orifice...
Text Solution
|
- Three grams of helium diffuses from a container in 15 min. The mass of...
Text Solution
|
- For two gases A and B with molecular masses MA and MB , it is observed...
Text Solution
|
- The kinetic energy of N molecules of O2 is x joule at - 123^@C. Anothe...
Text Solution
|
- Helium atom is two times heavier than a hydrogen molecule. At 298 K, t...
Text Solution
|
- Which of the following statement is not true?
Text Solution
|
- Four particles have speed 2,3,4 and 5 cm/s respectively. Their R.M.S s...
Text Solution
|
- If a gas expand at constant temperature :
Text Solution
|
- The heat capacity of the following gases at room temperature are such ...
Text Solution
|
- Which of the following expressions does not represent an ideal gas ?
Text Solution
|
- Above graph is plotted for CO2 at three different temperature, i.e., 2...
Text Solution
|
- At what temperature is the rms speed of hydrogen molecules the same as...
Text Solution
|
- The root mean square velocity of a gas is 'c'. if pressure of gas is d...
Text Solution
|
- By what factor does the average velocity of a gaseous molecule increas...
Text Solution
|
- Consider an ideal gas contained in a vessel. If the intermolecular att...
Text Solution
|
- A real gas at a very high pressure occupies
Text Solution
|
- The behaviour of a real gas is usually depicted by plotting compressib...
Text Solution
|