A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- X mL of H2 gas has effused through a hole in a container in 5 second....
Text Solution
|
- At identical temperature and pressure, the rate of diffusion of H2 ga...
Text Solution
|
- (plot of log P, where P = vapour pressure of liquid against 1/T) The s...
Text Solution
|
- Five molecules of a gas are moving with speed 1, 2, 3, 4, 5 km/sec. Wh...
Text Solution
|
- Most probable velocity of hydrogen molecules at T^@C is V0. At (2T+2...
Text Solution
|
- For a fixed mass of gas at constant pressure, which of the following s...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statements are not correct ?
Text Solution
|
- Let u(av),u(rms) and u(mp) respectively denote the average speed, roo...
Text Solution
|
- Which of the following statements are not correct ?
Text Solution
|
- Which of the following statements are not correct?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statements are not correct ?
Text Solution
|
- For an ideal gas under isobaric condition a graph between log V and lo...
Text Solution
|
- Let us consider 1 mol of gas. If It Is an ideal gas then< br> (Povers...
Text Solution
|
- Let us consider 1 mol of gas. If It Is an ideal gas then< br> (Povers...
Text Solution
|
- The root mean square velocity of hydrogen is sqrt5 times than that of ...
Text Solution
|
- The root mean square speed of N2 molecules in a sample at temperatur...
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
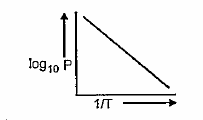 (plot of log P, where P = vapour pressure of liquid against 1/T) The slope of the line will be equal to :
(plot of log P, where P = vapour pressure of liquid against 1/T) The slope of the line will be equal to :