Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
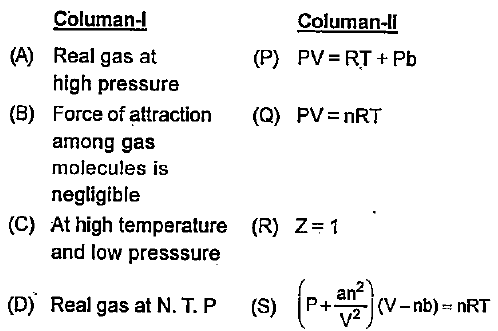
- Match Column-I with Column-ll
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
- 304 cm Hg pressure is equal to how many atm unit of pressure ?
Text Solution
|
- What is the pressure necessary to compress isothermally a 105dm^3 samp...
Text Solution
|
- A sample of gas at 0^@C and 1.00 atm pressure occupies 3.00 L. What ...
Text Solution
|
- At 400 K, the root mean square (rms) speed of gas X -(molecular mass =...
Text Solution
|
- A sample of a gas occupies 10 litre under a pressure of 1 atmosphere. ...
Text Solution
|
- One litre flask contains air, water vapour and a small amount of liqui...
Text Solution
|
- A sample of a gas occupies 10 litre under a pressure of 1 atmosphere. ...
Text Solution
|
- . 3.7 g of a gas at 25^@C occupied the same volume as 0.184 g of hydr...
Text Solution
|
- Write the formula of indian saltpetre.
Text Solution
|
- Two containers A and B have the same volume. Container A contains 5 mo...
Text Solution
|
- For 10 minutes each, at 27^@C , from two identical holes nitrogen and...
Text Solution
|
- The density of steam at 100.0^@C and 1x10^5 Pa is 0.6 kgm^(-3) Cal...
Text Solution
|
- Calculate molecular diameter of He if b (van der Waals’ constant for v...
Text Solution
|
- The critical constant for water are 374^@C, 218 atm and 0.0566 L mol...
Text Solution
|
- Calculate the pressure exerted by 5 mole of CO2 in one litre vessel a...
Text Solution
|
- According to Charles’ law :
Text Solution
|