A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- Calculate the pressure exerted by 5 mole of CO2 in one litre vessel a...
Text Solution
|
- According to Charles’ law :
Text Solution
|
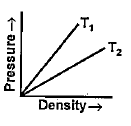
- Figure shows graphs of pressure versus density for an ideal gas at two...
Text Solution
|
- . A gas in an open container is heated from .27^@C to 127^@C. The frac...
Text Solution
|
- If there is no intermolecular force, what will be the volume of 4.5 kg...
Text Solution
|
- If the pressure of (N2,H2) mixture in a closed vessel is 100 atmospher...
Text Solution
|
- The heat capacity of the following gases at room temperature are such ...
Text Solution
|
- If one mole of a monatomic gas (gamma=5/3) is mixed with one mole of a...
Text Solution
|
- The rate of diffusion of NH3 is 3.32 times faster than that of an unkn...
Text Solution
|
- The Maxwell- Boltzmann distribution law of molecular speeds is graphic...
Text Solution
|
- The kinetic energy of N molecules of O2 is x joule at - 123^@C. Anothe...
Text Solution
|
- At what temperature will hydrogen molecules have the same kinetic ener...
Text Solution
|
- The ratio of average speed of an oxygen molecule to the rms. speed of ...
Text Solution
|
- At 27^@C the ratio of root mean square speeds of ozone and oxygen is:
Text Solution
|
- The temperature of an ideal gas is increased from 140 K to 560 K. If.a...
Text Solution
|
- The average molecular speed is greatest in case of a gas sample of:
Text Solution
|
- The root mean square speed of an ideal gas in a closed container of fi...
Text Solution
|
- The root mean square speed of the molecules of diatomic gas is u. When...
Text Solution
|
- A certain gas diffuses from two different vessels A and B. The vessel ...
Text Solution
|
- At 47^@C and 16.0 atm, the molar volume of NH3 gas is about 10% less t...
Text Solution
|