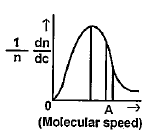
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- If one mole of a monatomic gas (gamma=5/3) is mixed with one mole of a...
Text Solution
|
- The rate of diffusion of NH3 is 3.32 times faster than that of an unkn...
Text Solution
|
- The Maxwell- Boltzmann distribution law of molecular speeds is graphic...
Text Solution
|
- The kinetic energy of N molecules of O2 is x joule at - 123^@C. Anothe...
Text Solution
|
- At what temperature will hydrogen molecules have the same kinetic ener...
Text Solution
|
- The ratio of average speed of an oxygen molecule to the rms. speed of ...
Text Solution
|
- At 27^@C the ratio of root mean square speeds of ozone and oxygen is:
Text Solution
|
- The temperature of an ideal gas is increased from 140 K to 560 K. If.a...
Text Solution
|
- The average molecular speed is greatest in case of a gas sample of:
Text Solution
|
- The root mean square speed of an ideal gas in a closed container of fi...
Text Solution
|
- The root mean square speed of the molecules of diatomic gas is u. When...
Text Solution
|
- A certain gas diffuses from two different vessels A and B. The vessel ...
Text Solution
|
- At 47^@C and 16.0 atm, the molar volume of NH3 gas is about 10% less t...
Text Solution
|
- Consider following equations : PV =nRT U(rms)=sqrt(3RT)/M R1/R2=...
Text Solution
|
- Energy of sublimation of solid helium is much lower than that of ice b...
Text Solution
|
- If the mean free-path of gaseous molecule is 60 cm.at a pressure of 1x...
Text Solution
|
- The ratio (a/b) (the terms used in van der Waals’equation) has the uni...
Text Solution
|
- A gas obeys the equation of state P(V-b) = RT (The parameter b is a co...
Text Solution
|
- Which of the following statements on critical constants of gases are c...
Text Solution
|
- lf Xm, Xp and Xv represent mole fraction, pressure fraction and volume...
Text Solution
|