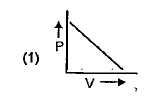
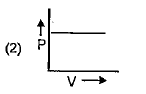
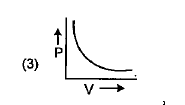
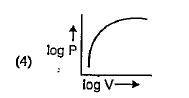
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- Equation of Boyle’s law is
Text Solution
|
- An ideal gas
Text Solution
|
- Among-the plots of P versus V at constant temperature as given below, ...
Text Solution
|
- The real gas will approach the ideal behaviour at
Text Solution
|
- When an open vessel is heated from 300 K to 500 K, what percentage of ...
Text Solution
|
- If pressure is tripled and temperature (in kelvin) is halved, the volu...
Text Solution
|
- Which of the following gases are heavier than air?
Text Solution
|
- Which of the following statement(s) is/are correct ?
Text Solution
|
- If a gas expand at constant temperature :
Text Solution
|
- The kinetic energy of one mole of a gas is given by the expression K.E...
Text Solution
|
- Write the names of the monomers of Glyptal.
Text Solution
|
- The van der Waals' constants of a gas are a=0.687dm^2mol^(-2)b=0.0226d...
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|
- Which of the following is/are true?
Text Solution
|
- Boyle temperature is the temperature at which the gas obeys Boyle’s la...
Text Solution
|
- Which of the following statements are incorrect ?
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|