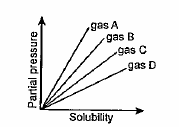
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-STATES OF MATTER (GASES & LIQUIDS)-QUESTION BANK
- Equal masses of H2O2 and methane have been taken in a container of vo...
Text Solution
|
- Density of carbon monoxide is maximum at
Text Solution
|
- From the given graph at constant temperature, which gas has the least ...
Text Solution
|
- If average velocity of a sample of gas molecules at 300 K is 5 cm s^(...
Text Solution
|
- For gaseous state, if most probable speed is denoted by C^**, average ...
Text Solution
|
- The rate of diffusion of methane is twice that of x. The molecular mas...
Text Solution
|
- At what temperature will the RMS velocity of SO2 be the same as that ...
Text Solution
|
- Write the Vander Walls equation for n'mole of the real gas.
Text Solution
|
- If P,T, p and R represent pressure, temperature, density and universal...
Text Solution
|
- Maximum deviation from ideal gas is expected from
Text Solution
|
- Write the Vander Walls equation for n'mole of the real gas.
Text Solution
|
- At what temperature will the RMS velocity of SO2 be the same as that ...
Text Solution
|
- The compressibility factor for a real gas at high pressure is
Text Solution
|
- Which of the following is correct at freezing point ?
Text Solution
|
- The ratio of diffusion of hydrogen and helium gas is
Text Solution
|
- A gas is heated through 1^@C in a closed vessel and so the pressure in...
Text Solution
|
- 50 mL of each gas A and gas B take 150 and 200 s respectively for effu...
Text Solution
|
- A certain gas takes three times as long to effuse out as helium. Its m...
Text Solution
|
- For real gases van der Waals’ equation is written as (p+(an^2)/V^2)(V-...
Text Solution
|
- During the. evaporation of liquid
Text Solution
|