Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-MOLE AND STOICHIOMETRY-II-QUESTION BANK
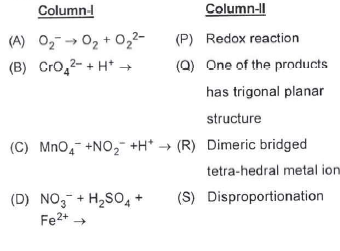
- Match Column-I with Column-ll
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
- Match Column-I with Column-ll
Text Solution
|
- 1.9 gm of CH3Bry has the same number of atoms as in 0.6 gm of H2O. The...
Text Solution
|
- 2.0g of polybasic organic acid(Molecular wt. = 600) required 100 mL of...
Text Solution
|
- A mixture contains 1.0 mole each of NaOH, Na2CO3 and NaHCO3. When half...
Text Solution
|
- What happens when xenon and fluorine are reacted in the ratio 1:20 res...
Text Solution
|
- A 2.76g impure sample of copper ore is dissolved and Cu^(2+) is titrat...
Text Solution
|
- The volume of 0.2M solution of MnO4^(-) which will react with 50.0 ml...
Text Solution
|
- Titration of 0.2121g of pure Na2CO4(134g mol^-1) require 43.31 ml of K...
Text Solution
|
- 1.44g pure FeC2O4 was dissolved in dil. H2SO4 and solution diluted to ...
Text Solution
|
- 0.592 g of calcium oxalate was dissolved in dilute acid and the soluti...
Text Solution
|
- 10 mL of a blood sample (contains calcium oxalate) is dissolved in aci...
Text Solution
|
- What is molality of equimolar mixture of water and ethanol ?
Text Solution
|
- 0.56 g of limestone was treated with oxalic acid to give CaC2O4. The p...
Text Solution
|
- A, B and C have oxidation number of +6, -2 and -1 respectively. What w...
Text Solution
|
- 5.7 g of bleaching powder was suspended in 500 ml of water. 25 ml of t...
Text Solution
|
- A solution of 0.1 M KMnO4 is used for the reaction : s2O3^(2-)+2MnO4^-...
Text Solution
|
- In aqueous alkaline solution, two electron reduction of HO2^- gives
Text Solution
|
- Assuming complete ionization, same moles of which of the following com...
Text Solution
|