A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-S-BLOCK ELEMENTS AND THEIR COMPOUNDS, HYDROGEN AND ITS COMPOUNDS-QUESTION BANK
- The order of solubility of lithium halides in non-polar solvents follo...
Text Solution
|
- A metal M readily forms water soluble sulphate, and water insoluble hy...
Text Solution
|
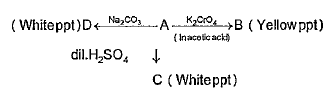
- If A is the metallic salt, them the white ppt. of D must be of
Text Solution
|
- A metal X on heating in nitrogen gas gives Y, Y on treatment with H2 O...
Text Solution
|
- Out of LiH, MgH2 and CuH
Text Solution
|
- Under what conditions of temperature and pressure, the formation of at...
Text Solution
|
- TiH1.73 is an example of
Text Solution
|
- The maximum possible number of hydrogen bonds a water molecule can for...
Text Solution
|
- Water obtained by purification of organic ion exchange resins is :
Text Solution
|
- In which of the following reactions does water act as an oxidising age...
Text Solution
|
- Which of the following is not true ?
Text Solution
|
- Which of the following is not correct regarding electrolytic preparati...
Text Solution
|
- The hybridisation state and oxidation state of two oxygen atoms in H2 ...
Text Solution
|
- Decolourisation of acidified potassium permanganate occurs when H2 O2 ...
Text Solution
|
- Decomposition of H2 O2 is accelerated by
Text Solution
|
- An aqueous solution of hydrogen peroxide ions is
Text Solution
|
- The species that does not contain peroxide ions is
Text Solution
|
- When same amount of zinc is treated separately with excess of sulphuri...
Text Solution
|
- Chemical A is used for water softening to remove temporary hardness. A...
Text Solution
|
- H2 O2rarr2H^oplus+O2+2e^(-)=-0.68V The above equation represents whi...
Text Solution
|