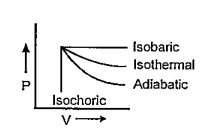
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- The standard enthalpies of formation of CO2(g) and HCOOH(l) are -393.7...
Text Solution
|
- Which of the following is a state function as well as an extensive pro...
Text Solution
|
- If the system involves gaseous substance, then pressure volume work W(...
Text Solution
|
- If the system involves gaseous substance, then pressure volume work W(...
Text Solution
|
- DeltaG of the system alone provides a criterion for the spontaneity of...
Text Solution
|
- DeltaG of the system alone provides a criterion for the spontaneity of...
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- The enthalpy of neutralisation of oxalic acid by a strong base is -25....
Text Solution
|
- 4.48 L of an ideal gas at STP requires 12 calories to taise its temper...
Text Solution
|
- An ideal gas is taken through the cycle A to B to C to A as shown in f...
Text Solution
|
- One mole of an ideal gas at 300 K is expanded ‘ isothermally from an I...
Text Solution
|
- 1 mole of an ideal gas is allowed to expand isothermally at 27^@C unti...
Text Solution
|
- 1 mol of an ideal gas undergoes reversible isothermal expansion from a...
Text Solution
|
- 1 mole of an ideal gas undergoes reversible isothermal expansion from ...
Text Solution
|
- Two litre of N2 at 0^@C and 5 atm pressure are expanded isothermally a...
Text Solution
|
- A 1.0 mol of ideal gas, initially at 10 atm and 300 K Is allowed to ex...
Text Solution
|
- A 1.0 mol of ideal gas, initially at 10 atm and 300 K Is allowed to ex...
Text Solution
|