Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
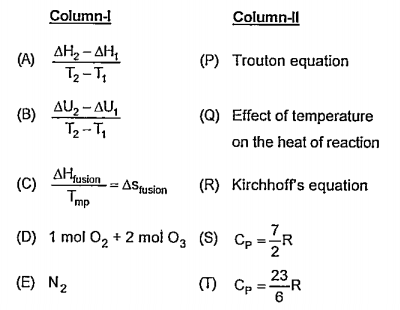
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- The enthalpy of neutralisation of oxalic acid by a strong base is -25....
Text Solution
|
- 4.48 L of an ideal gas at STP requires 12 calories to taise its temper...
Text Solution
|
- An ideal gas is taken through the cycle A to B to C to A as shown in f...
Text Solution
|
- One mole of an ideal gas at 300 K is expanded ‘ isothermally from an I...
Text Solution
|
- 1 mole of an ideal gas is allowed to expand isothermally at 27^@C unti...
Text Solution
|
- 1 mol of an ideal gas undergoes reversible isothermal expansion from a...
Text Solution
|
- 1 mole of an ideal gas undergoes reversible isothermal expansion from ...
Text Solution
|
- Two litre of N2 at 0^@C and 5 atm pressure are expanded isothermally a...
Text Solution
|
- A 1.0 mol of ideal gas, initially at 10 atm and 300 K Is allowed to ex...
Text Solution
|
- A 1.0 mol of ideal gas, initially at 10 atm and 300 K Is allowed to ex...
Text Solution
|
- A sample of argon gas at 1 atm pressure and 27^@C expands reversibly a...
Text Solution
|
- One mole of diatomic ideal gas undergoes a cyclic process ABC as shown...
Text Solution
|
- Calculate, the DeltaH at 85^@ for the reaction : Fe2O3(s) + 3H2 to 2...
Text Solution
|
- The standard heats of formation at 298 K for CCI4(g),H2O (g), CO2 (g) ...
Text Solution
|
- Show that the reaction CO(g) + 1/2 O2(g) to CO2(g) at 300 K is spontan...
Text Solution
|
- An isolated system is that system in which
Text Solution
|
- For a cycile process the condition(s) is/are
Text Solution
|