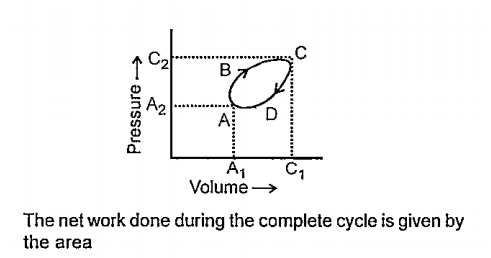
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- When a monoatomic gas undergoes an adiabatic process, its tempreature ...
Text Solution
|
- A monoatomic gas X and a diatomic gas Y, both initially at the same te...
Text Solution
|
- A thermodynamic system undergoes a cyclic change as represented in the...
Text Solution
|
- If w1, w2, w3 and w4 are work done in isothermalm, adiabatic, isobaric...
Text Solution
|
- Calculate DeltaH for this reaction, H2(g)+O2(g) rarr H2O2(g) B(H-H)=...
Text Solution
|
- How much heat is absorbed in complete reaction of 3.00 g of SiO2 with ...
Text Solution
|
- Standard enthalpies of formation of O3, CO2, NH3 and HI are 142.2, -39...
Text Solution
|
- What should be the amount of heat released when an aqueous solution co...
Text Solution
|
- The difference between the heats of reaction at constant pressure and ...
Text Solution
|
- The value of DeltaH(comb) of four gases C2H6, C2H4, C2H10, CH4 are giv...
Text Solution
|
- A solution of 500 mL of 0.2 M KOH and 500 mL of 0.2 M HCl are mixed an...
Text Solution
|
- The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 2.0 k...
Text Solution
|
- Given enthalpy of formation of CO2(g) and CaO(s) are -94.0 kJ and -152...
Text Solution
|
- H2(g)rarr2H(g), DeltaH=104.2 kcal. The bond energy of H-H bond is
Text Solution
|
- Which compound will absorb the maximum amount of heat when dissolved i...
Text Solution
|
- Heat evolved in the reaction, H2+Cl2rarr2HCl is 182 kJ. Bond energies ...
Text Solution
|
- For which change DeltaH ne DeltaU?
Text Solution
|
- The enthalpies of combustion of carbon and carbon monoxide are -393.5 ...
Text Solution
|
- A particular reaction has DeltaH =+250 kJmol^-1, which of the followin...
Text Solution
|
- Combustion of octand takes place in an automobile engine. The homogene...
Text Solution
|