A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- The heat liberated when 1.89 g of benzoic acid is burnt in a bomb calo...
Text Solution
|
- 1 mole of NH3 gas at 27^@C is expanded in reversible adiabatic conditi...
Text Solution
|
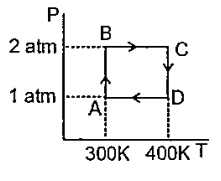
- Two moles of Helium gas undergos a cyclic process as shown in figure. ...
Text Solution
|
- Under the same conditions how many mL of 1 M KOH and 0.5 M H2SO4 solut...
Text Solution
|
- Heat of neutralisation of oxalic acid is -106.7 kJ mol^-1 using NaOH. ...
Text Solution
|
- If a and b are arbitrary extensive variables then
Text Solution
|
- During an adiabatic reversible expansion of an ideal gas
Text Solution
|
- Which is/are irreversible process(es)?
Text Solution
|
- Which of the following is/are thermodynamically stable?
Text Solution
|
- Which of the following expressions represent(s) work done by the isoth...
Text Solution
|
- For the adiabatic expansion of an ideal gas
Text Solution
|
- Which of the following is/are correct?
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|
- Which of the following reactions do(es)n't represent the standard stat...
Text Solution
|
- In a reaction DeltaH and DeltaS both are more than zero. In which of t...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|