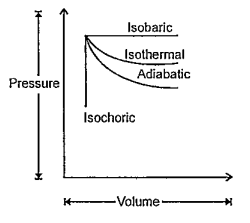
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- CP for monoatomic gas is
Text Solution
|
- Molar heat capacity is heat required to raise the temperature of one m...
Text Solution
|
- Observe the following graphic representation of four basic thermodynam...
Text Solution
|
- Match Column-I with Column-II
Text Solution
|
- Match Column-I with Column-II
Text Solution
|
- 2 g of an organic compound is burnt completely in a bomb calorimeter a...
Text Solution
|
- When a current of x ampere from a 20.9 V battery is used for 4.0 s to ...
Text Solution
|
- When 5.0 ml of 0.1 M HCI solution is mixed with 5.0 ml of a 0.1 M NaOH...
Text Solution
|
- Calculate the work done by the reaction (in L. atm) Fe(s)+H2SO4(aq)rar...
Text Solution
|
- Calculate entropy change when 10 moles of an ideal gas expands reversi...
Text Solution
|
- A gas present in a cylinder filled with a frictionless piston expands ...
Text Solution
|
- Work done in expansion of an ideal gas from 4 litre to 6 litre against...
Text Solution
|
- Calculate the maximum work done in expanding 16 g of oxygen at 300K an...
Text Solution
|
- One mole of an ideal monoatomic gas is heated at constant pressure fro...
Text Solution
|
- One mole of an ideal gas is heated at constant pressure from 0 o C t...
Text Solution
|
- Calculate DeltaH^@ for 2Al+Fe2O3rarr2Fe+Al2O3 given that standard heat...
Text Solution
|
- The heat liberated on complete combustion of 7.8 g benzene is 327 kJ. ...
Text Solution
|
- Calculate heat of combustion of benzene at constant pressure at 27^@C....
Text Solution
|
- The enthalpy change involved in the oxidation of glucose is -2880 kJ m...
Text Solution
|
- The heat of formation of ethane is -20.3 kcal. Calculate the bond ener...
Text Solution
|